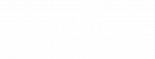FWO grants and other projects
FWO grants and other projects
Acrylate-Terminated Urethane-Based Hydrogel Precursors for Meniscus Tissue Engineering
Özge Begüm Akalin
Promotor: Prof. Peter Dubruel
Meniscus refers to cartilage tissue in the knee and it consists of two semilunar fibrocartilage discs, either lateral or medial. Both provide structural integrity to the knee when it undergoes tension and torsion. The purpose of the meniscus is to act as a stabilizer, shock absorber and space filler between the bones of the knee joint. Adequate and distinctive mechanical properties are necessary for a proper menisci function since it needs to endure constant pressure and endless, intense impacts on the joints. The blood flow of the meniscus is from the periphery to the central and there is no flow in the central region. The presence of blood flow divides the meniscus in three sections. Outer part, middle part and inner part are called as red zone, red-white zone and white zone, respectively. Mostly, tears of the meniscus particularly in the inner part need to be surgically corrected. However, this can cause to osteoarthritic degradation and eventual total knee joint replacement. Providing a meniscal scaffold that compensates biocompatibility and mechanical properties of the native tissue could delay or even prevent the formation of osteoarthritis. A tissue engineered, patient specific 3D-printed meniscus with optimized mechanical and biocompatible properties can be the key solution for meniscal injuries.
The aim of this project is to investigate the application of photopolymerized acrylate-terminated urethane-based polyethylene oxide (PEO) hydrogel precursors (AUP) as porous scaffolds for meniscal tissue engineering. This approach enables to create patient-specific scaffolds with tunable physical properties not only via altering the design but also via altering the backbone molecular weight of the AUPs.
Future research on the AUP scaffolds will be performed including drug release and in vivo testing to enhance the development of a tissue engineered, patient specific 3D-printed meniscus.
Targeting tendon-bone junction regeneration: development of a gradient scaffold.
Dr. Nele Pien
Promotors: Prof. Sandra Van Vlierberghe, Prof. Catharina De Schauwer
The tendon-bone (T-B) junction is the transition zone from soft to hard tissue, prone to acute and overuse injuries in both human and equine athletes. Functional integration between tendon and bone remains a major challenge after injury, as the presence of inflammation affects the healing process, resulting in the formation of scar tissue. Tissue engineering strategies should be explored to support T-B regeneration. To this end, the characteristic gradient structure of the junction, i.e. the spatial distribution with a gradual decrease in collagen fiber organization while proteoglycan and mineral content is gradually increasing, should be mimicked.
To develop such a functional scaffold, tailor-made photo-crosslinkable and biodegradable polymers (i.e. urea/urethane-based norbornene-endcapped precursors and thiolated decellularized extracellular matrix) will be synthesized and characterized. Then, the developed materials will be 3D printed into a continuous scaffold exhibiting a gradient in mechanical properties. Finally, by encapsulating undifferentiated mesenchymal stem cells (MSCs) during 3D-printing along with their differentiation factors, the MSCs will gradually differentiate towards the desired phenotypes.
Undifferentiated MSC will secrete anti-inflammatory factors, which is beneficial to counter the inflammation observed immediately after injury. Once the MSC are differentiating, trophic factors will be produced, which is beneficial to support T-B regeneration.
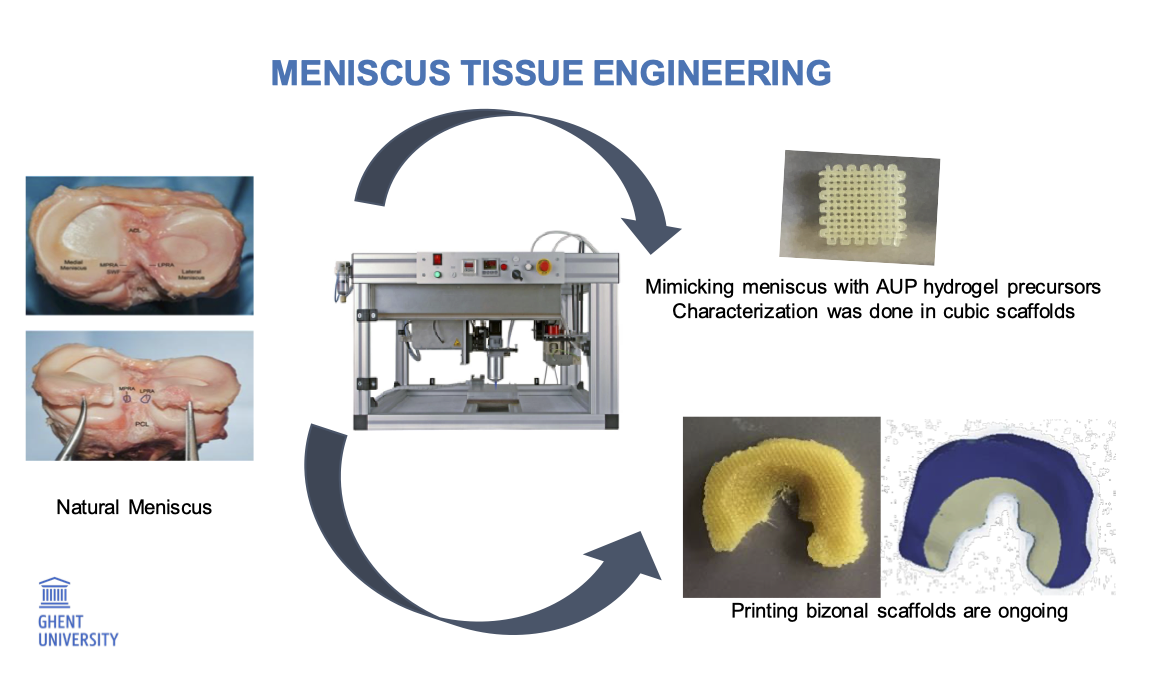
Introducing smart polymers in the field of corneal endothelial tissue engineering: solving a blinding disease
Lobke De Vos
Promotors: Prof. Peter Dubruel, Prof. Sandra Van Vlierberghe
Eye diseases are responsible for a huge economical burden globally, but are also associated with a drastic decrease in quality of life. Some of these diseases are associated to the loss of transparency of the window of the eye, namely the cornea. The cornea is the outermost part of the eye and is composed out of 3 different cell layers. The innermost layer, the corneal endothelium, maintains critical corneal hydration. Upon ageing, disease or trauma, this cell layer can be damaged to such an extent that the cornea swells and loses its transparency, which leads to blindness. Currently, the only treatment consists of full or partial transplantation of a donor cornea. Unfortunately, the supply does not meet the demand by far since only 1 donor is available per 70 patients. To overcome this limitation, the present project aims to develop a synthetic alternative that allows the efficient transplantation of healthy cells towards the site of tissue defect. To this end, biodegradable membranes will be developed using a combination of smart polyesters with shape memory effects, in combination with gelatin derivatives that mimic the cellular environment. These carriers will be seeded with cells, to allow transplantation to the site of tissue defect. Furthermore, the membranes will be analysed in depth both for mechanical properties as in vitro behaviour prior to in vivo animal studies.
Smart polymers to tackle a blinding disease: Corneal endothelial tissue engineering
Jasper Delaey
Promotors: Prof. Peter Dubruel, Prof. Sandra Van Vlierberghe
Eye diseases are responsible for a huge economical burden globally, but are also associated with a drastic decrease in quality of life. Some of these diseases are associated to the loss of transparency of the window of the eye, namely the cornea. The cornea is the outermost part of the eye and is composed out of 3 different cell layers. The innermost layer, the corneal endothelium, maintains critical corneal hydration. Upon ageing, disease or trauma, this cell layer can be damaged to such an extent that the cornea swells and loses its transparency, which leads to blindness. Currently, the only treatment consists of full or partial transplantation of a donor cornea. Unfortunately, the supply does not meet the demand by far since only 1 donor is available per 70 patients. To overcome this limitation, the present project aims to develop a synthetic alternative that allows the efficient transplantation of healthy cells towards the site of tissue defect. To this end, biodegradable membranes will be developed using a combination of smart polyesters with shape memory effects, in combination with gelatin derivatives that mimic the cellular environment. These carriers will be seeded with cells, to allow transplantation to the site of tissue defect. Furthermore, the membranes will be analysed in depth both for mechanical properties as in vitro behaviour prior to in vivo animal studies.
Development of a smart diagnostic antimicrobial hydrogel-based wound dressing
Manon Minsart
Promotors: Prof. Peter Dubruel
Prof. Dr. Ir. A. Mignon (KU Leuven)
Burn wounds lead to an estimated 300 000 deaths per year worldwide. Infection remains the main cause of morbidity and mortality in burn victims. Moreover, diagnosis of wound infection is not always straightforward. One of the critical issues concerning commercial dressings today is their uncontrolled release of bioactive compounds, e.g. antimicrobials. This FWO-SB project focuses on the development of a ‘smart’ burn wound dressing using patented PEG-based acrylate-endcapped urethane-based hydrogel precursors (AUPs), which can be cross-linked with UV-A irradiation and thus form 3D hydrogel networks. Their exceptional solid-state reactivity is particularly of interest for polymer processing through electrospinning. Electrospun fibre mats can provide an excellent environment to promote wound healing by mimicking the native extra-cellular matrix. The ability to incorporate diagnostic as well as antimicrobial compounds in these networks will be especially of interest in this research.
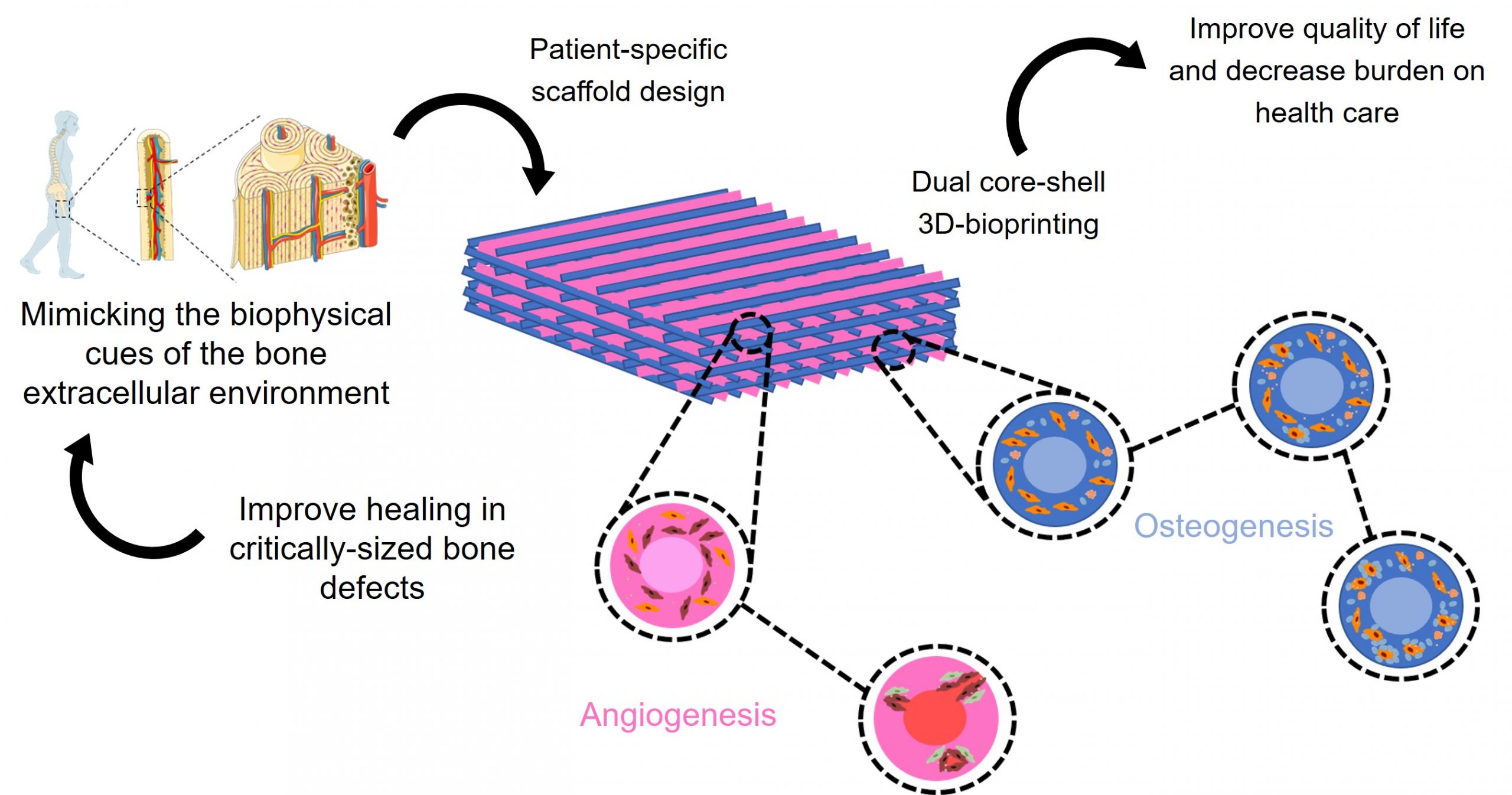
Capturing the biophysical cues of bone to stimulate regeneration in critical bone defects
Laurens Parmentier
Promotors: Prof. Peter Dubruel, Prof. Sandra Van Vlierberghe
Do you know someone who suffered from a bone defect that had problems to heal after disease, trauma or surgical intervention? The answer is probably yes since bone is the second most commonly tissue transplanted worldwide and is primarily used in critical bone defects in which bone is unable to repair itself through bone remodelling. In order to develop an antimicrobial, patient-specific substitute material that guides bone formation, favours vascularisation and hence stimulates bone repair without commonly reported side-effects associated with conventional grafts such as pain, infections and an enhanced immune response, this project looks at mimicking bone through various biophysical cues such as composition, architecture, mechanical properties and degradation. Moreover, this mimicry will be realised hierarchically on both the macroscopic load-bearing scale of the scaffold fitting the bone defect and the non-load bearing scale of the extracellular matrix substrate providing the appropriate environment for cell adherence, proliferation and differentiation. Dual core-shell 3D bioprinting will be used to fabricate the scaffold consisting of an osteogenic and an angiogenic filament. Characterisation will be performed to target the incorporation of the bone mimicking biophysical cues into the bone substitute material. The proposed material solution offers great potential to tackle current issues towards the development of a bone substitute material that can be used in critically sized bone defects which is of utmost importance given their prevalence, their associated morbidity and their socio-economic implications.
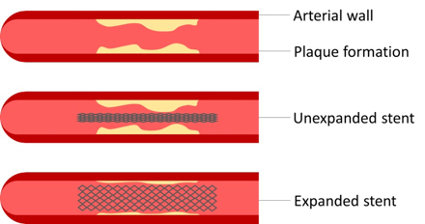
Shape memory polymers based on poly(alkylene terephthalate)s for an improved cardiovascular stent
Lenny Van Daele
Promotors: Prof. Peter Dubruel, Prof. Sandra Van Vlierberghe
This project involves the use of polymers for cardiovascular applications, more specifically for stents. We aim to develop new polyesters that exhibit shape memory behaviour. Using this polymer technology and surface modifications, we aim to develop a new stent that outperforms the stents that are currently on the market. To this end, an improved endothelialisation and reduced thrombogenicity is within our objectives. Finally, using 3D-printing technology, a personalized stent can be produced which will fit each patient perfectly.
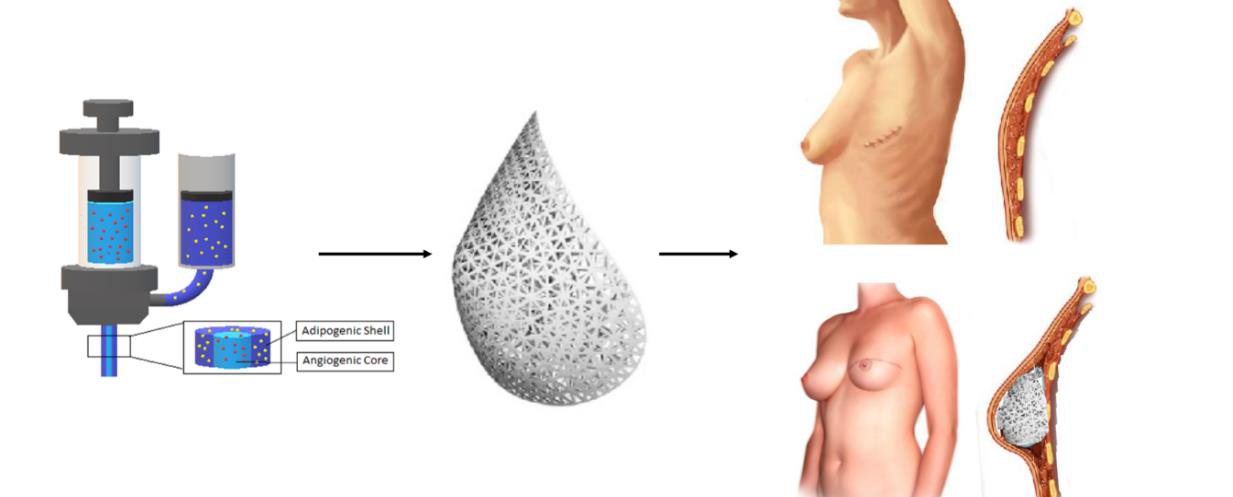
Development of 3D printed core/shell hydrogels towards patient specific breast reconstruction
Lana Van Damme
Promotors: Prof. Sandra Van Vlierberghe
Prof. Phillip Blondeel
Nowadays, breast implants and micro-surgical free tissue transfer are the most popular procedures to repair soft tissue defects resulting from vastectomies/lumpectomies following breast cancer. With breast cancer being the most common cancer affecting women worldwide, there is a clinical need for reconstructive strategies addressing current drawbacks and limitations.
The development of biomimetic materials able to promote proliferation and adipogenic differentiation have gained increasing attention for adipose reconstructive purposes. The aim of the current research project includes
(1) the development of biodegradable, gelatin-based hydrogel building blocks with photo-polymerizable groups focusing on step growth polymerization which can be applied as starting materials for the fabrication of 3D constructs;
(2) 3D printing of constructs with an adipogenic shell and an angiogenic core;
These structures will then be assessed on biocompatibility via in vitro and in vivo assays as well as differentiation potential. The project outcome could offer a shift towards a patient-specific 3D printed, minimally invasive reconstructive approach, that can potentially be safer and more cost-effective.
Acrylate-endcapped urethane-based polyethylene glycol polymers for post-surgical chronic rhinosinusitis treatment
Jan-Philip Zegwaart
Promotors: Prof. Peter Dubruel, Prof. Sandra Van Vlierberghe
Chronic rhinosinusitis (CRS) is a condition of perpetual inflammation of the sinuses in the nasal cavities, which affects about 15 % of the US and the European population. When drug treatments fail, surgical intervention is required. Draf III surgery is the final available option. During this procedure, a drainage pathway (neo-ostium) at the frontal sinus is created. Nonetheless, ostium closure still occurs in 22% of the cases.
The aim of the project is to develop a frontal sinus wound care solution using acrylate-endcapped urethane-based polymers (AUPs). AUPs are a promising type of macromonomers that allow the development of tailor-made polymer networks, including hydrogels, upon photo-crosslinking.
Within the project, 15 different types of AUPs were developed (subdivided into 4 categories). The AUPs are biocompatible, whereby the swelling and the material strength can be tailored. A second part of the research is focused on converting the AUPs into SLA resins, opening unprecedented 3D printing capabilities.
Future research on these AUP hydrogels entails drug-elution and in vivo testing, to advance the development of a superior frontal sinus wound care solution.
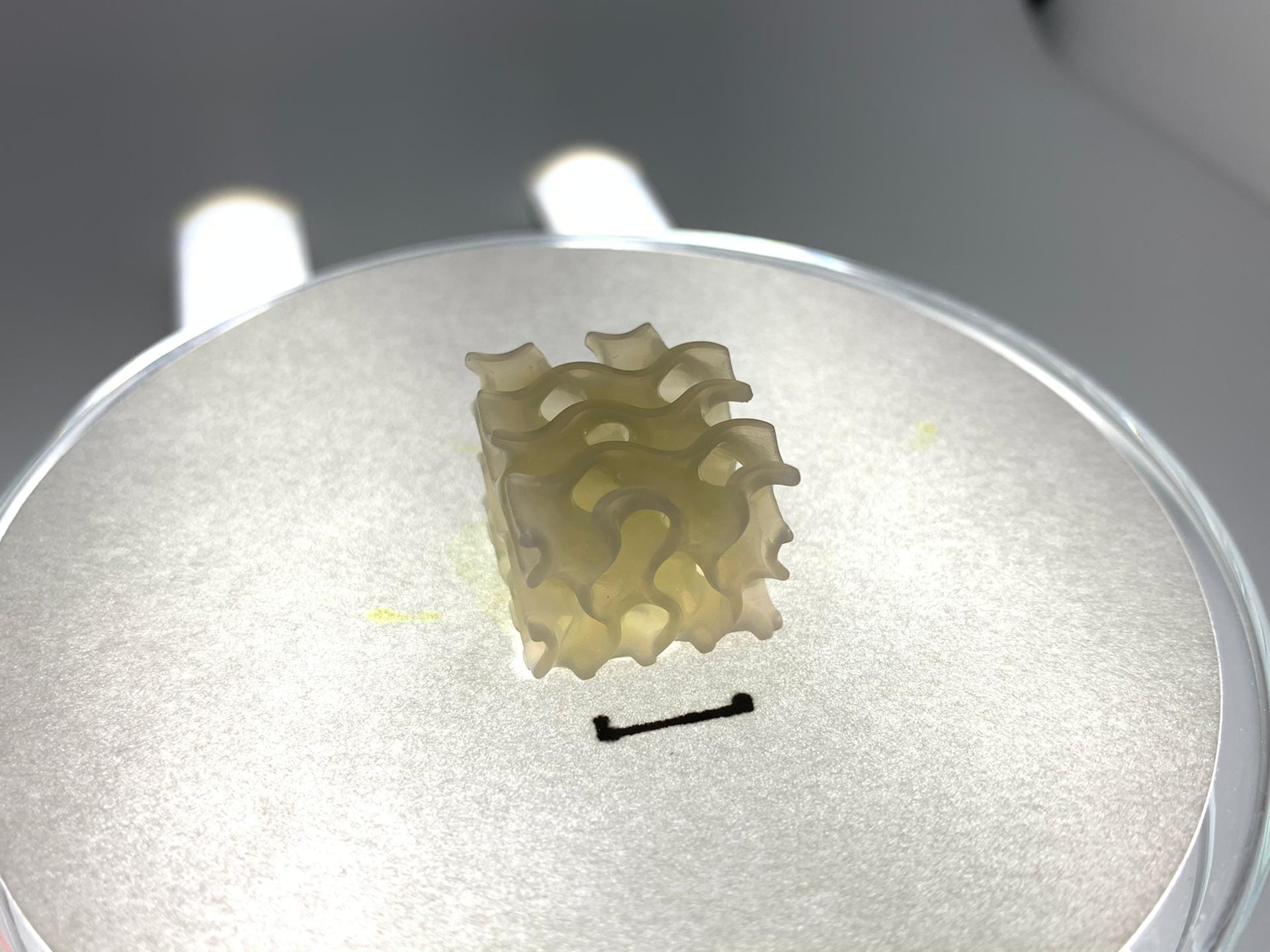
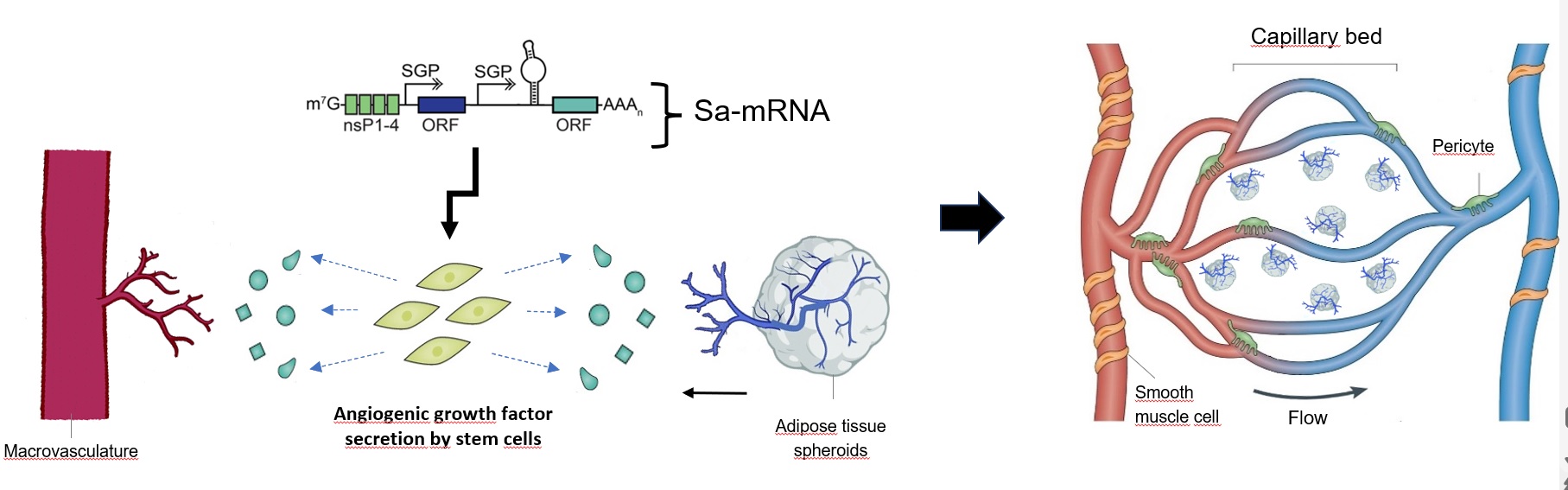
Biofabrication of vasculature for organ engineering and bypass
grafting
Florian Vanlauwe
Promotor: Phillip Blondeel (ZAP, Plastic and Reconstructive Surgery)
There is a large deficit of donor organs in transplant surgery. Moreover, autologous free tissue transfer in reconstructive surgery is imperfect due to invasive harvesting of tissue and the long duration and complexity of the operation. The biofabrication of organs is a promising strategy to diminish the shortage of donor organs and to resolve the shortcomings in free autologous tissue transfer. To biofabricate large organs, the integration of vasculature is essential. This comprises a microvascular network that overcomes the diffusion limits for oxygen and nutrients and macrovasculature that permits fast perfusion of the whole construct when connected to an in vitro or in vivo circulatory system. In this research project, a vascular model for organ engineering will be created that comprises both macro- and microvasculature. The macrovasculature will be 3D bioprinted with a novel vascular biomaterial consisting of a mixture between gelatin and elastin. To form the microvascular network, angiogenesis from the macrovasculature towards vascularized microtissues will be stimulated using biomaterial modifications (eg. alterations in physico-chemical properties and 3D architecture) and saRNA technology. In contrast to the current state-of-the-art, this model will be suitable for direct connection to the host’s vasculature by surgical anastomosis, which will make the transplantation of large 3D bioprinted organs possible. Additionally, the biofabricated macrovasculature can be tested as a small diameter graft for bypass surgery.
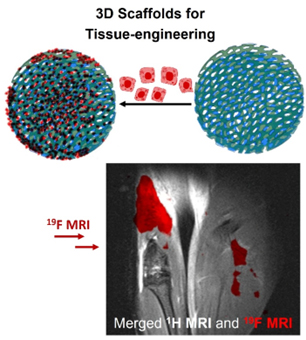
Thermoresponsive Poly[N-(2,2-difluorethylacrylamide)] 19F MRI Tracer-Based Materials as Potential Multipurpose Theranostics
Kristyna Koluchova
Promotor: Prof. Sandra Van Vlierberghe
Macromolecular 19F MRI tracers which contain fluorine in their backbones, are a relatively new and promising category of 19F MRI tracers. Polymer-based tracers can contain a high concentration of chemically equivalent fluorine atoms and their solubility and biodistribution can be tuned by a careful selection of monomer types constituting the polymer. In general, such macromolecular structures can be designed by combining various components, resulting in advanced materials serving an immense range of purposes, from smart drug/gene delivery systems (nanoparticles) to implants/gels for tissue engineering (hydrogels). By combining the therapeutic applications of polymer-based systems with the imaging modality of tracer, we create so called theranostic system (therapy-diagnostic).
In this project, we develop variety of 19F MRI tracers containing poly[N-(2,2-difluoroethyl)methacrylamide] (PDFEA). The resulting tracers will serve for a range of above-mentioned medical applications, while having the imaging properties. As a part of this project, we will include various types of post-polymerization/crosslinking modification (introducing a targeting group, stimuli-responsive group, etc.) to specify a medical application of the resulting tracer. Most of the project will be focused specifically on gelatine crosslinking with the DFEA monomer, which will provide a 3D printable hydrogel scaffold, with the possibility of simultaneous in vivo tracking.
CustoMeat
Elly De Vlieghere & Indi Geurs
Promotor: Prof. Sandra Van Vlierberghe
Cellular agriculture is a promising avenue for sustainably contributing to the growing food demand. In this respect, cultured or in vitro meat receives increasing attention. Yet, there is currently no scientific evidence that animal myofibers equivalent to meat as a nutrient dense, highly structured food, can be made in vitro. This proposal address several existing hurdles. A
major challenge is to obtain a suitable cell type capable of both sufficient proliferation and robust differentiation. Muscle adult progenitor cells have a very good differentiation capacity, yet weak proliferation capacity, whereas mesenchymal and embryonic stem cells display the opposite behavior. First, we will compare and enhance the proliferation and differentiation capacity of these three cell types, resulting in an informed choice of cell type for myofiber generation. Secondly, we will design a ‘smart matrix’, including food-grade components that contribute to texture, flavor and a good nutritional profile. This involves a proper choice of materials which is mutually dependent on the technological approaches to create a 3D structure. Physicochemical stability of, and cellular adhesion and survival on these new matrices will be investigated. The best performing smart matrices will be tested on their potential to support muscle cell differentiation. These insights will be subsequently used to increase muscle fiber size and to study in vitro muscle development in a 3D architecture. In addition, strategies for stimulating the build-up of contractile proteins will be investigated. Finally, public acceptance, consumer attitudes, perceptions and intentions, as well as impact on sustainability will be assessed.
Collaborating Researchers:
- Universiteit Gent, Laboratory for Animal Nutrition and Animal Product Quality (LANUPRO) – Prof. Stefaan De Smet
- Universiteit Gent, nutriFOODchem – Prof John Van Camp, dr. Charlotte Grootaert
- Universiteit Gent, Food Structure & Function Group (FSF) – Prof. Koen Dewettinck, dr. Daylan Tzompa Sosa
- Universiteit Gent, Veterinary stem cell research - Prof. Catharina De Schauwer
- Universiteit Gent, Dpt. of Virology, Parasitology and Immunology –, Prof. Bert Devriendt
- Universiteit Gent, Agro-food Marketing and Consumer Behavior (AFMCB) – Prof. Wim Verbeke, dr. Yung Hung
- Universiteit Gent, Sustainable Systems Engineering (STEN) – Prof. Jo Dewulf, dr. Thuy Trang Nhu
- Universiteit Gent, Polymer Chemistry and Biomaterials Group (PBM) – Prof. Sandra Van Vlierberghe
- Universiteit Gent, Research Group Medical Cell Biology – Prof. Jolanda Van Hengel
- KULeuven, Campus Kortrijk, Unit Development and Regeneration – Prof. Lieven Thorrez
- Universiteit Gent, Food2Know
- Project coordinator – dr. Elly De Vlieghere
- PhD students – Indi Geurs, Chloë Deelkens, Maria Olenic, María Ignacia Rodríguez Escobar
Smart Polymer-based Wound Dressings with Diagnostic and Antimicrobial Capabilities
Fatma Nalan Cetin
Promotors: Prof. Sandra Van Vlierberghe, Prof. Arn Mignon (KU Leuven)
Chronic wounds are nowadays still a serious concern in healthcare and require new and improved treatments.One of the major issues present in the open wound management is bacterial infection.To tackle this issue, within the proposed project, a novel and ‘smart’ polymer-based wound dressing will be developed which maintains the moisture balance and holds additionally both diagnostic and antimicrobial properties. Nanovesicles will be created whereby part will be loaded with a self-quenched dye and part with an antimicrobial compound and will break open in a controllable way when required.Thenanovesicles will be incorporated in a ‘nature-derived’,photo-crosslinked polymer based wound dressing.The wounddressing will be processed usingco-axial electrospinning to createnanofibers with a core-shell morphology. The dressings will be optimized to tune theirswelling and mechanical properties as well as their diagnostic andantimicrobial capabilities.
The project involves the Polymer Chemistry and Biomaterials research group of UGentand the Biomaterials and Tissue Engineering research group of KU Leuven.

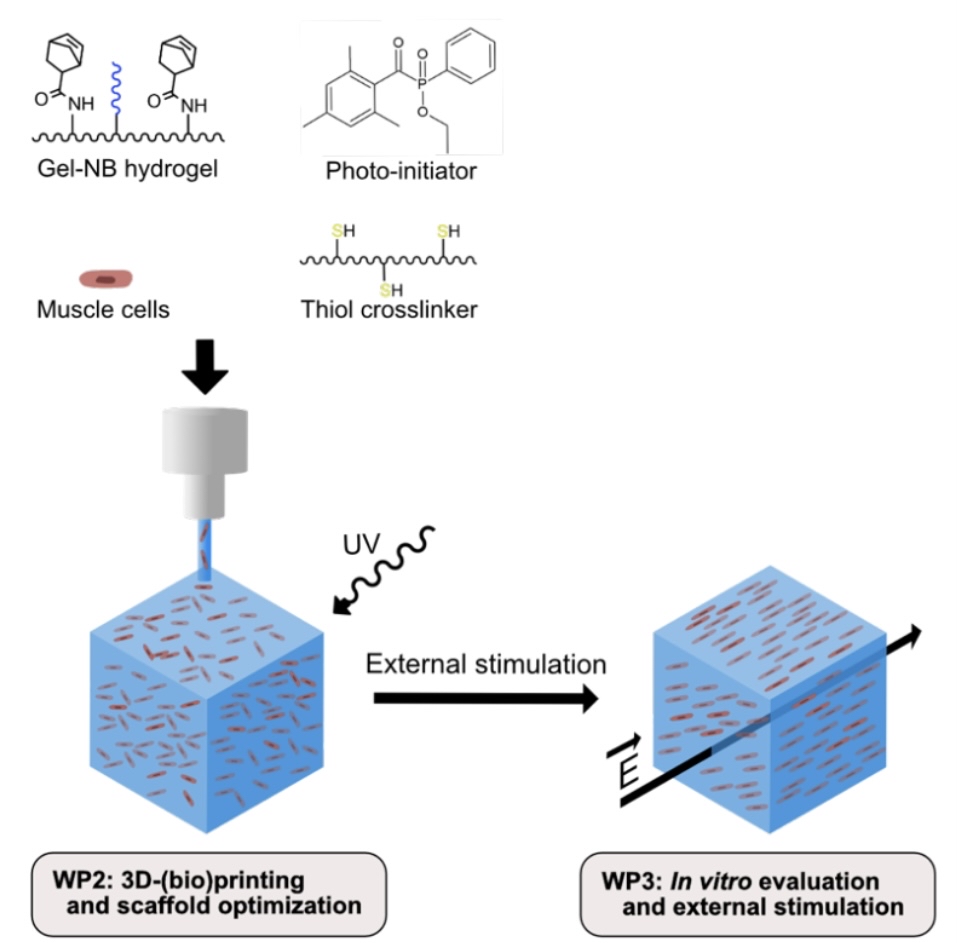
Volumetric muscle loss, caused for instance by traffic accidents or tumor resection, has dramatic consequences for a patient’s quality of life. In the context of tissue engineering, several conductive muscle scaffold materials have been reported. However, most of these conductive materials do not support cell encapsulation, hence preventing bioprinting of muscle scaffolds. The current PhD aims to address the above-mentioned shortcomings by developing conductive and piezo-ionic bioinks via ‘grafting from’ polymerization approaches onto gelatin. For crosslinking purposes, gelatin norbornene (Gel-NB) is selected due to its favorable step-growth thiol-ene photo-crosslinking mechanism, in the presence of a thiolated gelatin as crosslinker. The conductive Gel-NB hydrogel will be obtained via oxidative polymerization of pyrrole. In addition, a piezo-ionic Gel-NB hydrogel will be developed by grafting anionic or cationic (meth)acrylates/(meth)acrylamides using RAFT polymerization. The functionalized and characterized Gel-NB hydrogels will be processed into porous scaffolds by means of 3D-bioprinting. Cell alignment, proliferation and differentiation will be evaluated for the conductive versus piezo-ionic cell-encapsulating scaffolds. In this regard, in vitro electric field and mechanical stimulation will be exploited.
3D-Bioprinting of Conductive and Piezo-Ionic Gelatin Hydrogels for Skeletal Muscle Tissue Engineering
Joeri Van Meerssche
Promotors: Prof. Sandra Van Vlierberghe, Prof. Toon Verstraelen (CMM), dr. Jelle Vekeman (CMM)
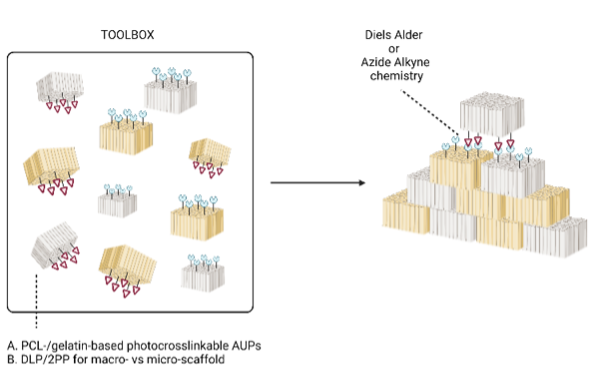
Exploiting a chemical toolbox towards LEGO-inspired tissue engineering
Bo Van Durme
Promotors: Prof. Sandra Van Vlierberghe
Tissue injury and organ failure due to disease, injury and developmental deficits have become a major health problem for billions of people worldwide. Currently, surgical interventions, organ transplantation, or implantable devices offer a solution to address these issues. As a result, there exists an increasing demand for donor organs. However, the shortage of organ donors leads to ever growing transplant waiting lists and hence to longer waiting times. A possible solution to address this organ shortage problem is the field of tissue engineering (TE). Although significant progress has been realized in recent years, there remain shortcomings related to current TE approaches. The most important shortcoming is the scaffold mimicry towards native tissue. One single biomaterial combined with one specific additive manufacturing technique cannot fulfill all requirements to mimic the physico-chemical complexity of native tissue which is a strict necessity for successful tissue engineering. Hence, it is of paramount importance to develop a universal type of chemistry which allows to connect (‘glue’) 3D-printed scaffolds regardless of the material type (i.e. natural and synthetic) or printing technique applied. The current PhD project will focus on the development of a combinatory approach in which macro- and micro-structures will be combined into one single construct in an attempt to mimic the physico-chemical and morphological complexity of tissues to the greatest extent possible. To this end, both a thermoplast (PCL) and a hydrogel (gelatin) will be selected as starting materials for construct development. The design strategy entails the incorporation of complementary chemical functionalities in the scaffold materials, through which printed constructs can be linked following 3D printing.

Over the past decade, biodegradability properties of various polymer materials have gained a lot more attention. Previously, polymers were being advertised as an exceptionally durable product, which could not degrade in nature. At first, this was understood as an enormous advantage. However, around 1960, an incredible shift in awareness could be noticed and from this moment, non-biodegradability was considered as a disadvantage as well. Furthermore, the biodegradability in biomedical applications, such as implants, is of utmost importance as well. At the moment, only permanent implants are being used. However, as the implant remains indefinitely in the body, it could cause long-term inflammatory reactions. Therefore a very promising alternative approach is tissue engineering (TE). TE aims to restore tissue defects via a temporary scaffold in order to stimulate tissue regeneration while simultaneously providing mechanical support. However, a fundamental requirement in the application of TE is to generate synthetic bioresorbable networks that mimic the targeted tissue. Consequently, it is crucial to gain control over the specific biodegradation time, in order to employ them in various (biomedical) applications.
The objective of this PhD is to develop and investigate thiol-ene photo-crosslinked networks, based on various synthetic, bioresorbable polyesters (PLA, PGA, PLGA, PCL, PTMC and PHB). The chemical design of each polyester network will be altered, in order to address an entire platform of biodegradation rates. Considering that the required degradation rate is strongly dependent on the specific application, the ability to tune the degradation time of the network is of utmost importance.
In a second part of the PhD project, each of the investigated two-component systems will be exploited in the context of three different light-based 3D-printing techniques. Digital light processing (DLP), volumetric additive manufacturing (VAM) and two-photon polymerization 3D-printing (2PP) will be practiced, in order to obtain polyester scaffolds with high resolution and printing time.
Exploiting chemical design to create a photo-crosslinkable polyester platform serving biomedical applications
Astrid Quaak
Promotors: Prof. Sandra Van Vlierberghe
Development of a biodegradable bone graft for maxillofacial reconstruction
Quinten Thijssen
Promotors:
Prof. Sandra Van Vlierberghe
Maxillofacial Surgeon Robin Willaert (Maxillofacial Surgery Dept. of UZ Leuven)
Maxillofacial reconstruction still remains a challenge in the 21st century since the currently available treatments such as autologous bone grafts and titanium implants are very invasive and pose a high risk for infections, often resulting in implant removal.
Therefore, this FWO-SB project focusses on the development of a patient specific and biodegradable implant. A combination of functionalized photo-reactive polymeric building blocks will be investigated as candidate for maxillofacial reconstruction using 3D-printing techniques.
Other Grants and Projects

Smart biomaterials towards minimally invasive breast reconstruction
Coralie Greant
Promotor: Prof. Sandra Van Vlierberghe
The development of biomaterials for adipose regeneration has gained increasing attention as a result of the exponential growth of adipose tissue reconstructions performed in health care. In addition to cosmetic considerations, these reconstructions are also attempted for women undergoing lumpectomies after breast cancer treatment, which is highly relevant as breast cancer is the most prominent cancer striking women worldwide. Recently, an increasing interest has emerged from material engineers to develop materials for adipose tissue engineering and breast reconstruction in particular, addressing the existing limitations. New biomaterial-related approaches should ideally aim for a more predictable outcome, an improved cost-effectiveness and minimal invasiveness. It is exactly there that the current project will come into play by combining patien-specific 3D printing with biodegradable shape memory polymers to replace the currently used invasive, artificial expander. These biodegradable smart polymers will then act as a support for autologous tissue injected into the pores.
BioMeniscus – Development of an artificial meniscus through 3D polymer printing
Martina Meazzo
Promotors: Prof. Peter Dubruel
Catherine Van Der Straeten (UZ Gent)
Prof. Fabrizio Barberis (Genoa University)
The menisci are fibrocartilaginous structures that contribute to distribute compressive forces during joint movement, to static weight bearing, joint lubrication, joint stabilization and proprioception. For this reason, research into meniscus repair has been the recipient of particular interest from the orthopaedic and bioengineering communities because current repair techniques are only effective in treating lesions located in the peripheral vascularized region of the meniscus but not for non-vascularized region.
The primary method nowadays for treatment is still partial meniscectomy, which commonly results in the progressive development of osteoarthritis. This drawback has shifted research interest toward the fields of bioengineering and biomaterials.
The goal of BioMeniscus is to investigate the development of a novel meniscus implant based on a new type of polymer that mimics more accurately as possible the complicated meniscus structure.
One of the crucial features of a scaffolds is adequate mechanical properties. Therefore, one of the principal aims is to mechanically characterize them though different mechanical tests (compression test, tensile test, DMA, fatigue test, shear test etc). Some of these tests will be performed in collaboration with Genova University.


Acrylate-endcapped urethane-based polymers for the development of iodine-based antibacterial wound dressings
Georgios Misiakos
Promotor: Prof. Sandra Van Vlierberghe
The World Health Organization estimates that globally, burn injuries are responsible for
180,000 deaths every year. The leading cause of death in these cases is sepsis caused by infections. Iodine-based antibacterials are a potential successor to silver due to a lack of bacterial resistance and allergies, an efficacy against biofilms and covering a broad spectrum against microorganisms, however reapplication is needed every 12 to 24 hours.
Scaffolds with an ability to withhold iodine and release it gradually, would eliminate the major drawback of this antibacterial approach, which is the goal of this project. To this end, the manufacturing of acrylate-endcapped urethane-based polymer (AUP) sheets through electrospinning is employed. AUPs offer the novel ability of crosslinking in the solid state which opens up new pathways in the development of materials and the tuning of their properties. In addition, the method of electrospinning enables the creation of highly porous scaffolds with the potential to have sufficient mechanical robustness, while having the ability to uptake iodine and release it in a favorable timeframe.
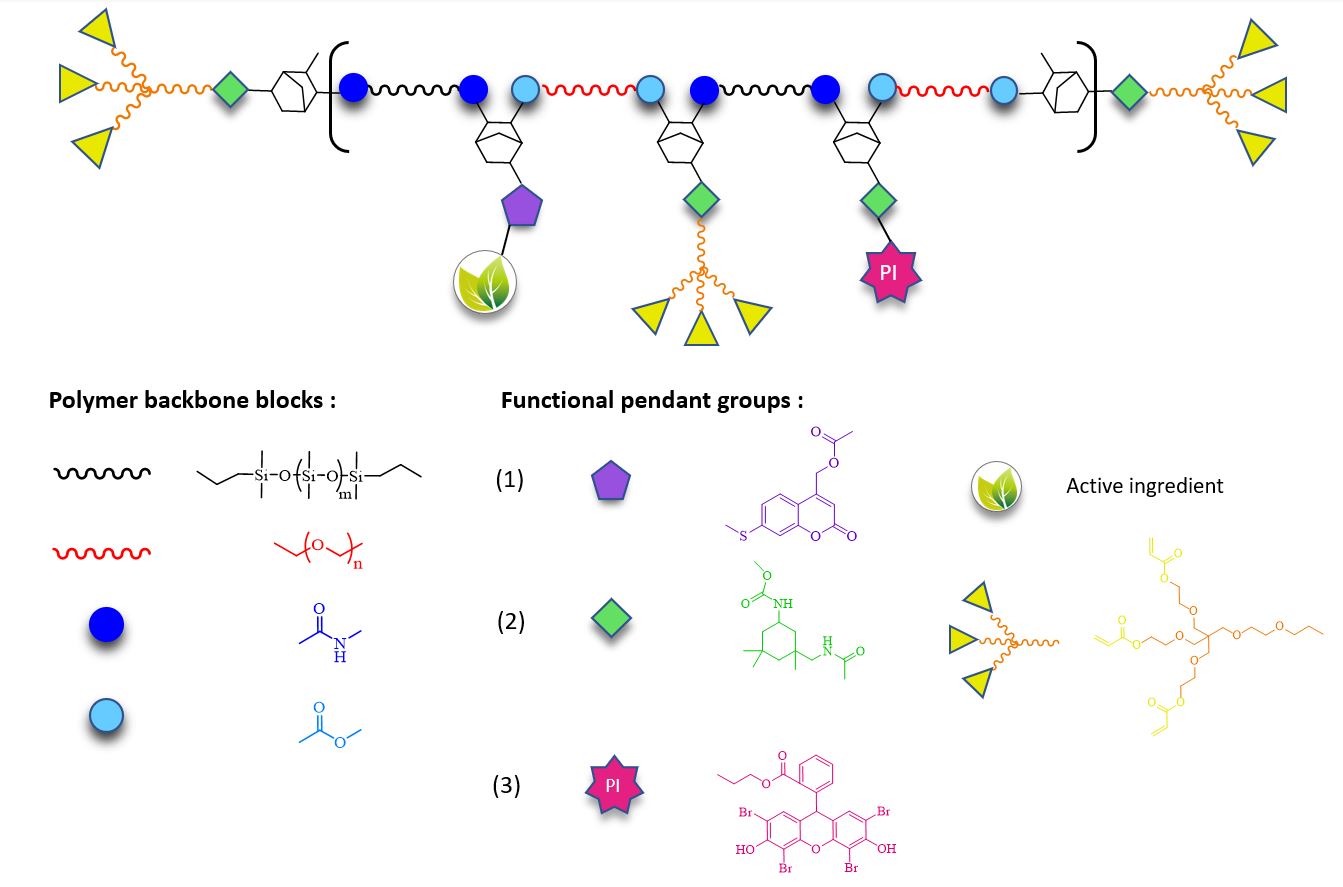
Development of photo-crosslinkable, light-responsive polymers for cosmetic applications
Gregorio Fantini
Promotors: Prof. Sandra Van Vlierberghe, Prof. Peter Bottenberg (Université Libre De Bruxelles)
The skin, being the largest organ strategically located at the interface between the external and internal environment, provides a barrier against numerous sources of potential stress including mechanical, chemical, biological or resulting from UV irradiation (which is usually associated with cutaneous aging phenomena arising from tissue photodamage). Treatment of such photoinduced tissue defects could be achieved combining the advantageous structural features responsible for the enhanced flexibility and spreadability of Si-containing polymers already employed in cosmetic formulations with a synthetic material able to undergo fast crosslinking upon exposure to visible light and functionalizable towards tailored applications (such as a cosmetic composition for skin tautening, wrinkle reduction and targeted skincare). This would pave the way towards the development of novel, multipurpose products serving the cosmetics market. While the treatment of photodamaged and/or aging tissues could be achieved upon in situ crosslinking with visible light-induced irradiation, subsequently a light-based stimulus of a different wavelength could trigger the photo-induced release of desired active substances with well-known anti-aging, antioxidant, UV-protective properties. To develop bioadhesive synthetic materials, polycondensation and post-polymerization functionalization are exploited in order to obtain novel well-defined block-co-polymers of polyether and polysiloxane chains bearing multiple reactive groups to be subjected to further modification towards the incorporation of coumarin-derived photo-cleavable active compounds, branched acrylate-based photo-crosslinkable functionalities and photo-initiators active in the visible range.
3D Biomimetic model of the host-microbiome small intestinal
ecosystem
Inez Roegiers
Promotors:
Prof. dr. ir. Tom Van de Wiele (CMET)
Prof. dr. Peter Dubruel (PBM)
dr. Tom Gheysens (PBM)
dr. Marta Calatayud Arroyo (CMET and Prodigest)
The 3D intestine project aims to develop an in vitro model resembling the small intestine microenvironment and architecture, including human microbiota and the host interface and thus, cover the technological gap of mimicking the small intestine ecosystem in vitro. Our model will allow the research community and industry sector to evaluate in vitro the oral bioavailability of drugs or food compounds and simultaneously assess the microbial, host and environment interactions. The combination of fundamental insights from microbiology, human physiology, cell cultures and reactor
technology will be integrated in a multidisciplinary approach to attain as the main deliverable a reproducible model with a broad range of applications. This model will provide quantitative parameters to evaluate not only host parameters (e.g. epithelial barrier function, immune response) but also functional and structural microbiome shifts. In addition to the commercial importance of this research, the project will also contribute significantly to the scientific understanding of microbial-human interactions and its relevance for health. The project maximizes expertise from many labs to ensure excellence in the science outcomes of the project. The applicability of this model to pharmaceutical and nutrition industry and also for basic research will generate new knowledge and also a highly applicable outcome.
The development of degradable implants for autologous breast reconstruction
Nicole Ritter
Promotors: Prof. Sandra Van Vlierberghe, Prof. Philip Blondeel
Breast cancer has the highest incidence rate among all cancers but ranks only fifth in mortality. Prevention and treatment frequently include the removal of a breast. It is thus essential to provide breast reconstruction methods that offer satisfying results to patients. Modern reconstruction techniques have drawbacks such as capsular contracture, complex microsurgery or multi-step procedures. Lipofilling suffers from the latter, in part due to the fragility of adipocytes. Additionally, most cases of breast reconstruction require skin expansion. Therefore, it would be beneficial to find ways in which the adipocytes from the lipoaspirate can be protected while also expanding the skin to allow for efficient lipofilling.
This research aims at developing and performing a pre-clinical evaluation of a novel polymer-based 3D-printed medical device to improve breast reconstruction after mastectomy with the reverse expansion surgical technique using lipofilling. This mammary expander should provide support, volume, and low-pressure zones. The implant will also degrade over a longer period of time, during which the lipofilling will have regenerated sufficient extra-cellular matrix to be viable and avoid resorption.